
Have you ever wondered how your TV remote, wall clock, or flashlight comes to life with just a small battery inside? Behind that tiny metal cylinder lies a fascinating process where chemical reactions create electric current. In this chapter of our Analog Electronics series, we’ll explore how a battery works — how it stores energy in chemical form and then converts it into electrical energy whenever you connect it to a circuit.
Inside a battery, there are three main parts: the anode, the cathode, and the electrolyte. The anode is usually made of zinc, the cathode is manganese dioxide (MnO₂), and the electrolyte is a chemical paste, such as potassium hydroxide (KOH), which contains mobile ions.
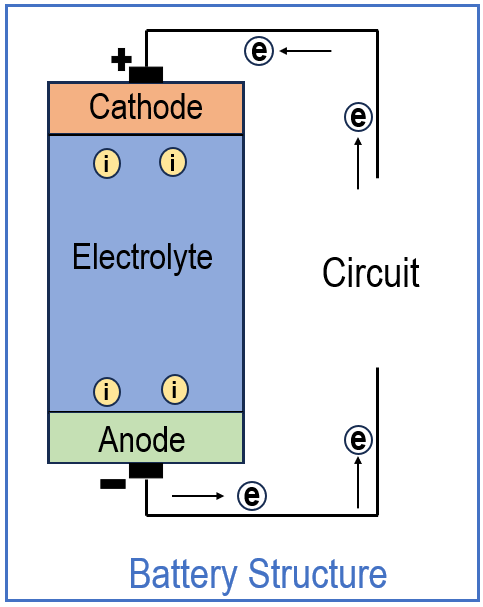
Zinc is chosen as the anode because of its low reduction potential. In simple words, zinc atoms prefer to lose electrons rather than gain them, making them an excellent source of electrons. On the other hand, the cathode is made of a material like MnO₂, which has a high reduction potential, meaning it readily accepts electrons. This contrast is what allows the battery to function.
What Happens When the Battery is Connected?
At the anode, zinc undergoes oxidation, meaning it loses electrons:
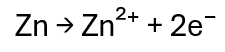
These electrons cannot pass through the liquid electrolyte. Instead, they leave the zinc electrode, flow through the external circuit, and power a connected device, such as a bulb. This flow of electrons through the wire is what we call electric current.
Electrons vs Ions — The Two Different Paths
Before going further, it’s important to understand the difference between electrons and ions, since both play key roles in how a battery works. Electrons are tiny subatomic particles with a negative charge, and they move very easily in metals like copper wires. Ions, on the other hand, are atoms or groups of atoms that have gained or lost electrons. If an atom loses electrons, it becomes a positively charged cation (like Zn²⁺). If it gains electrons, it becomes a negatively charged anion (like OH⁻).
Unlike electrons, ions are much larger and cannot move through metal wires. They can only migrate through the electrolyte, which is specially designed to let them move and maintain charge balance in the battery. So, while electrons flow outside the battery through the circuit, ions move inside the battery through the electrolyte. Both flows are necessary for the battery to function.
Think of it this way: electrons take the “outside road” through the wires to do work, while ions take the “inside road” through the electrolyte to keep the battery balanced. Without the electrolyte, charges would build up: the anode would become too positive with Zn²⁺ ions, and the cathode would become too negative with incoming electrons, eventually stopping the flow. The electrolyte prevents this by carrying ions to balance the charges.
Ion Movements Inside the Battery
When zinc gives off Zn²⁺ ions, these ions stay in the electrolyte near the anode. To maintain neutrality, OH⁻ anions from the electrolyte migrate toward the anode to neutralize the positive Zn²⁺ buildup. At the same time, other cations (like H⁺ or Mn-containing intermediates, depending on the chemistry) move toward the cathode, where electrons are arriving from the external circuit.
It’s important to note that Zn²⁺ ions do not travel to the cathode. Since the cathode is positive, Zn²⁺ ions are repelled and remain in the electrolyte. Meanwhile, other ions like OH⁻ move appropriately to balance charge. This way, the electrolyte ensures continuous reactions without direct zinc migration to the cathode.
Reduction at the Cathode
At the cathode, the electrons that traveled through the external wire finally arrive and participate in a reduction reaction. The MnO₂ at the cathode reacts with electrons and hydroxide ions from the electrolyte:
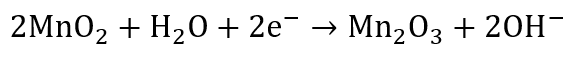
This reaction consumes the incoming electrons, allowing the circuit to remain continuous. Electrons outside the battery and ions inside the electrolyte work together, creating a seamless flow that powers devices.
Putting It All Together
Here’s the full picture:
- Anode (Zinc): Zinc atoms oxidize into Zn²⁺, releasing electrons into the external circuit.
- Electrons: Travel through the wire to do useful work, like lighting a bulb or running a motor.
- Electrolyte (KOH): Allows ions to move inside the cell so that charges don’t build up and reactions can continue.
- Cathode (MnO₂): Accepts electrons and undergoes reduction.
In short, the battery constantly converts chemical energy into electrical energy. Zinc releases electrons (oxidized), manganese dioxide accepts electrons (reduced), electrons travel outside, ions move inside, and together this system keeps the current flowing.
Conclusion
A battery is a simple yet brilliant device that converts chemical energy into electrical energy through redox reactions. At the anode, oxidation releases electrons; at the cathode, reduction consumes them. While electrons travel through the external circuit to power devices, ions move inside the electrolyte to maintain charge balance. Together, these movements keep the current flowing continuously.
In short, every battery — from the one in a flashlight to the one in a smartphone — operates on the same fundamental principle: chemical reactions driving the steady flow of electrons that power our world.
No comments:
Post a Comment