
In the fascinating world of electronics, semiconductors form the foundation of all modern devices — from the smallest microchips to the most complex integrated circuits. To understand how electronic components like diodes, transistors, and amplifiers work, it’s essential to first grasp what semiconductors are and how their conductivity can be controlled.
Semiconductors
Atoms may combine to form a solid crystalline material through covalent bonding. In silicon, each atom forms covalent bonds with four neighboring silicon atoms, creating a strong and stable crystal lattice. A pure silicon crystal, without any impurities, is called intrinsic silicon.
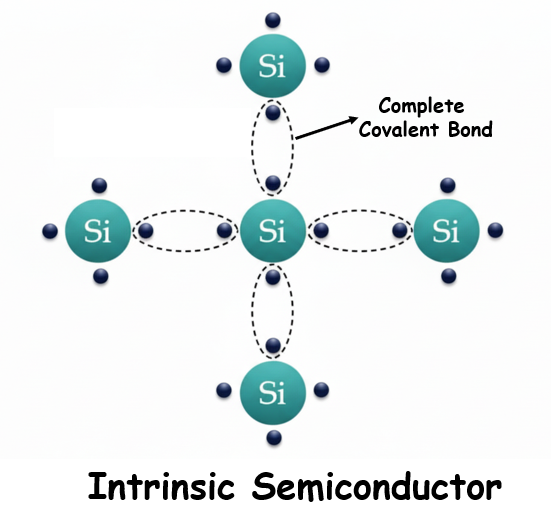
At room temperature, intrinsic silicon has very limited conductivity. Some valence electrons gain sufficient thermal energy to jump from the valence band to the conduction band. When this happens, free electrons are generated in the conduction band, and vacancies are created in the valence band. These vacancies are called holes.
If a voltage source is applied across intrinsic silicon, the thermally generated free electrons in the conduction band will move toward the positive terminal of the voltage source. This movement produces current in the material, which is called electron current. Simultaneously, holes in the valence band contribute to conduction. When an electron from a neighboring atom moves to fill a hole, it leaves behind a new hole. This movement of holes generates hole current.
Thus, intrinsic silicon shows conduction through both free electrons and holes, but the conductivity is very low.
Extrinsic Semiconductors
The conductivity of semiconductors can be greatly improved by a process called doping, in which controlled amounts of impurities are added to pure silicon. Depending on the type of impurity added, the resulting material can be either n-type or p-type semiconductor.
N-Type Semiconductor (Pentavalent Impurity)
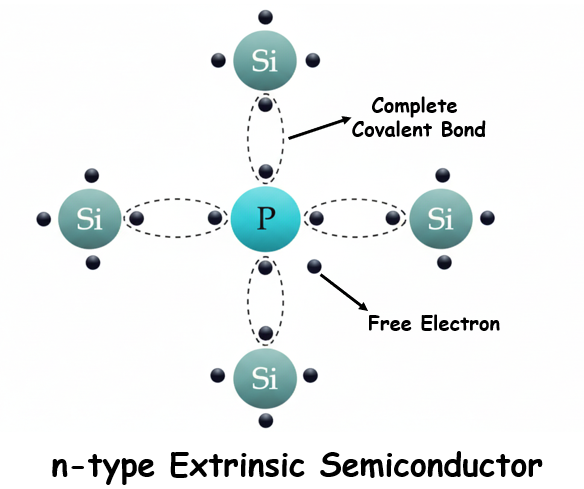
When a pentavalent impurity (an atom with five valence electrons, such as arsenic, phosphorus, bismuth, or antimony) is added to silicon, four of its valence electrons form covalent bonds with four neighboring silicon atoms. The fifth electron, however, does not participate in bonding and remains loosely bound to the dopant atom.
This extra electron requires very little energy (about 0.01 eV) to move into the conduction band, which is far less than the 1.1 eV required to break a silicon-silicon covalent bond. At room temperature, almost all these donor electrons are free to move, creating a large number of charge carriers without disturbing the crystal lattice.
As a result, n-type semiconductors exhibit much higher electrical conductivity at room temperature compared to intrinsic silicon, where only a few valence electrons can thermally jump to the conduction band. In n-type semiconductors, electrons are the majority carriers, while holes are the minority carriers.
P-Type Semiconductor (Trivalent Impurity)

When a trivalent impurity (an atom with three valence electrons, such as boron, indium, or gallium) is added to silicon, each dopant atom forms covalent bonds with three neighboring silicon atoms. This leaves one bond incomplete, resulting in the creation of a hole.
At room temperature, electrons from neighboring silicon atoms can move to fill this hole, which in turn creates a new hole at the position from where the electron moved. In this way, holes effectively move through the crystal lattice and act as charge carriers.
Thus, in a p-type semiconductor, holes are the majority carriers, while electrons are the minority carriers.
Summary
Intrinsic semiconductors are pure crystals with very low conductivity, where current is generated only by thermally excited electrons and holes.
Extrinsic semiconductors are doped with impurities to enhance conductivity.
- Pentavalent impurities create n-type semiconductors, where electrons are the majority carriers.
- Trivalent impurities create p-type semiconductors, where holes are the majority carriers.
Conclusion
Semiconductors are the backbone of modern electronics. By understanding the difference between intrinsic and extrinsic types, we unlock how materials can be engineered to conduct electricity in controlled ways — forming the base for diodes, transistors, and integrated circuits.
No comments:
Post a Comment